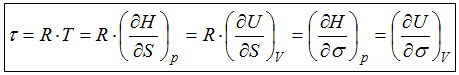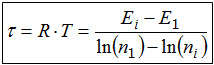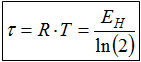|
A comparison of the two definitions shows that in both cases a quotient occurs. Because in both cases the numerator is a difference of energy values, we conclude that also the denominator indicates an equal quality in both cases. From the second definition it is clear that this quality is a difference of numbers. Of course the values of the "occupation numbers" must be natural numbers, because it is not possible to store only one half of a particle on the energy levels. The quality of a number is not lost by the logarithm. If the intensity phenomenon 'temperature' is described by RT* this would as well be indicated by the unit kJ/mol, because all intensive properties are described by quotients.
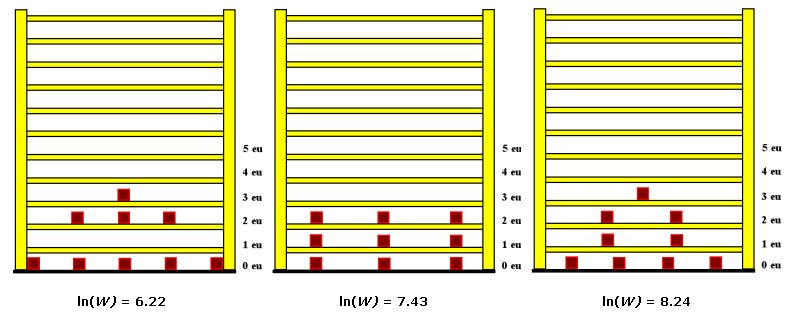
The stored energy is related to the numbers of microstates, which are accessible for the system or substance having that certain amount of stored energy. Under common temperature conditions the number of microstates is so immense that no human being is really able to get a vivid perception of such a number. For 100g common salt or 10 liters of Helium the values lie in the order of magnitude of: 103.1024.
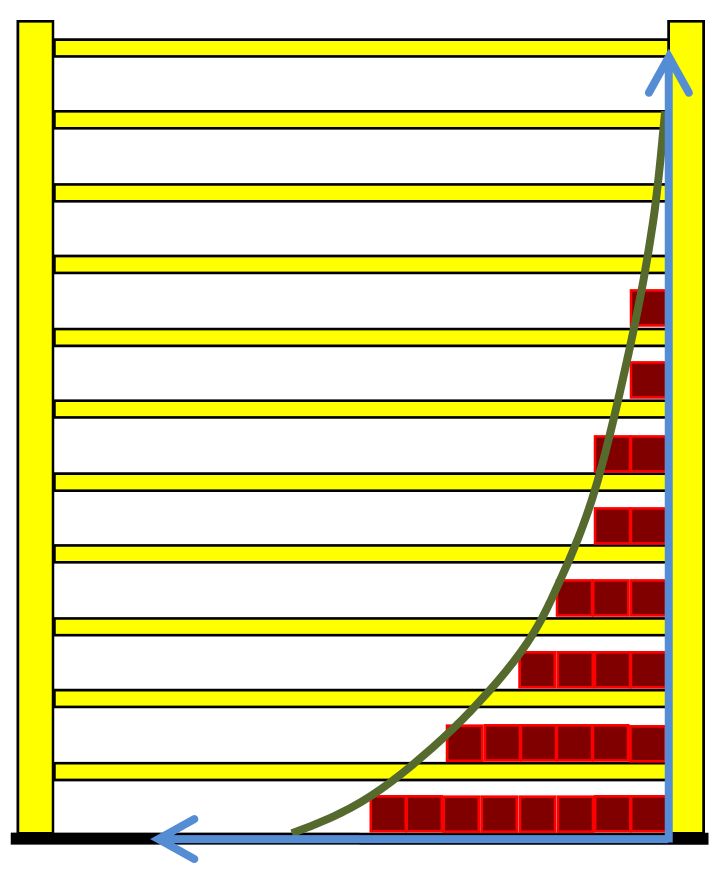
The number of microstates is strictly monotonic increasing with the number of occupied energy levels. So it is a correct simplification to say: entropy is a measure of the number of occupied energy levels. And at the same time this is a wise simplification because the number of occupied levels lies in a comprehensible order of magnitude. For 10 liters of Helium we find about 2000 occupied levels.
A mathematical peculiarity of the exponential function can be used to simplify the thermodynamic definition of the temperature on the basis of the energy quantization. This special feature is that, after constant changes of the independent variables (here the energy), the function values halve in half. This property is well known from the radioactive decay, where it is used to characterize the speed of the decay: since the independent variable for radioactive decay is the time, the half-life characterizes this phenomenon. In complete analogy, one can speak of the half-energy EH, ie, the additional energy distance Ei - E1 at which the occupation number ni of a level is halved in comparison to lowest level n1.
EH = Ei - E1
ni /n1 = 0,5 ⇒ ln(n1) - ln(ni) = ln(2).
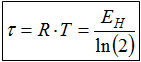
*(The difference between the Boltzmann constant k and the gas constant R consists only of one unit change. Correctly, in the case of unit conversion, a numerical value and a unit symbol should appear on both sides of the equality sign: 1 mol = 6,02214 . 1023 co. (Here, the unit symbol 'co' stands for 'countable objects'.)
This yields: k = 1,38066 . 10-23 JK-1co-1 = 8,31453 JK-1mol-1 = R.
For the understanding of the thermodynamic relationships, it does not seem to be helpful to change the symbol for the quantity value when changing a unit, since quantity values are independent of the units used. )
|