|
The basis for the three rules lies in the energy quantization, which can be calculated with the help of the Schrödinger equation. There are four different ways of storing thermal energy by basic chemical particles such as electrons, atoms, ions and molecules. They are called:
- Translational states
- Rotational states
- Vibrational states
- Elektronic states
(It is striking that only the latter type is not linked to the idea of a type of movement. Although quantum theory has led to different ideas about these "movements," the terms have nonetheless been preserved. Since the electronic states are thermodynamically and quantum chemically of little influence on the material behavior, they are not treated here.)
Quantum objects are located most of the time in their characteristic energy states, which can be described by the Schrödinger equation. These states do not last forever, but there are "lifetimes" for them. If this time period has expired, the quantum object changes to another state, which is energetically attainable for this object. Each change is associated with electromagnetic radiation, which is either absorbed or emitted, depending on whether the new state is higher or lower in energy. The transition processes can be spontaneous or induced.
During the lifetime of a state, the Schrödinger equation gives a space function for each state which is constant for the duration of the corresponding state throughout the space. That is why they are called stationary states. For electrons, the widely accepted designation "orbital function" is used for these functions. Spatial changes of a quantum object do not occur during the lifetime of this object, but only at the transitions.
Mathematical formulars for the distances between the
energy levels include the following terms:
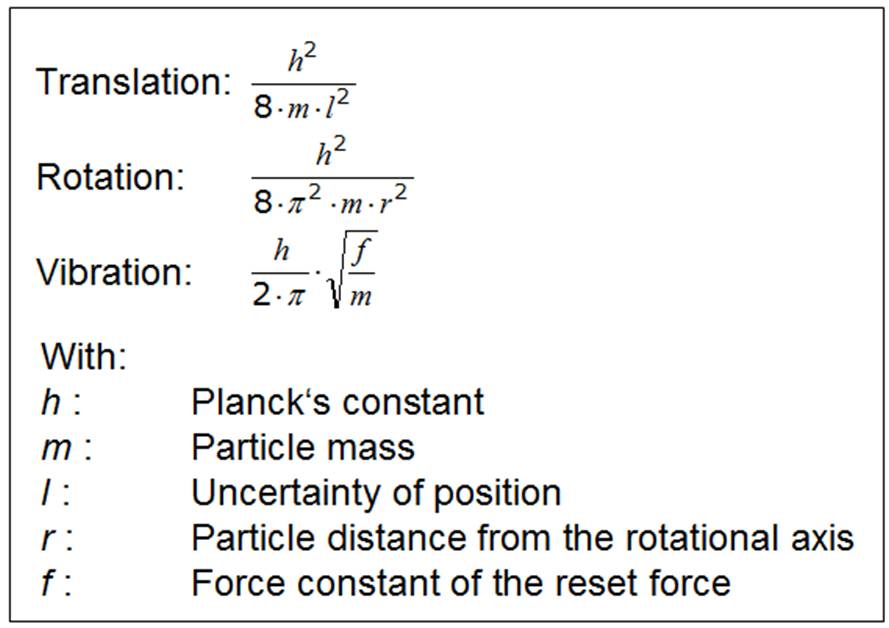
These relations determine the energetic position of the associated energy levels. They are called eigenvalues or energy eigenvalues, because in these relationships one sees that the characteristic properties of the particles are contained: geometry, restoring forces, and mass of the quantum objects.
Easily one recognizes the mass rule because the mass
always enters the denominators, thus a large mass leads to small level spacing.
You see the force rule for example in the fact that in the vibration-term the force
constant f is found in the counter or the uncertainty of position l arises in the
denominator of the translation-term. A small uncertainty of position is the result of a strong
force.
If a substance, a system, is heated by the open flame of a burner or by an electric heating plate, photons enter the system and are absorbed by the system. Thereby, particles of the system change from lower to higher energy levels.
The following animation shows such a process:
Animation: Thermal Work in the Shelf Model
The envelope curve of the occupation numbers becomes shallower, the half-value energy, a measure for the temperature of the system, increases. This process is referred to as the thermal work carried out by the burner or the heating plate on the system.
In this process, the level differences remain constant and only the particles are redistributed on their levels. If the particles change their levels, this is always associated with electrical alternating fields. Radiation is absorbed or emitted. The system changes from one Boltzmann distribution to another with a flat or steeper course, depending on absorption or emission. In order for particles to change their levels, electrical alternating fields are required. These need not necessarily be transmitted with electromagnetic radiation, but can also occur as a result of friction. (See 6.4 of this website)
If a system is compressed with a piston, the movement space of the particles also becomes smaller. The length of this space in the denominator enters the translation formula and causes the level distances to increase.
The following animation shows how this process can be imagined in the shelf model:
Animation: Mechanical Work in the Shelf Model
A piston can only affect the system with electrostatic interactions. In the case of the atoms of the piston as well as of the system, the negative charges of the atomic shells touch and abut. The electrostatic fields can not lift the particles to different levels, but only change the position of these levels. The occupancy numbers and the number of occupied levels remain constant and it is understandable that the entropy also remains constant.
|
