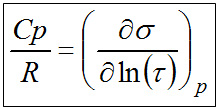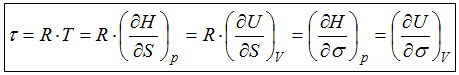3. The Fluid Model
|
In chapter 2 it was shown how the storage of thermal energy can be understood on a quantum theoretical basis. |
3.1. The Theoretical Fundamentals of the Fluid Model
|
The thermodynamic relationship between the molar entropy σm and the molar thermal capacity Cp can best be seen in the relationship given below
|
|
Since the logarithm function is monotonically increasing, the term ln(τ) also increases with increasing temperature. If the entropy σ is semi-logarithmic against the temperature, the thermal capacity can be determined as the slope (first derivative) in this diagram. Thermodynamics distinguishes four types of quantized energy storage. Because the chemical substances have four kinds of eigenvalues, there are also four types of energy stores, which belong to four types of energy states: translational, rotational, vibrational, and electron states. They behave differently when thermal or volume work on the substances is carried out. The fluid model is capable of adequately visualizing the different behavior, because it is able to convert quantum-chemically calculated values of the individual memories graphically. The Thermulation-II program calculates these values from the known spectroscopic data and can output these as numerical values, as Boltzmann distributions and as fluid model diagrams. |
|
Thermodynamically, the temperature is defined by the relation given below. |
|
|