Considering Chemical Bond Phenomena
|
Comparing the thermodynamic data of the halogens and the noble gases, taking into account the energy quantization, it becomes clear that for the halogens the additional storages for the thermal energy are due to the chemical bonding. While only the translational levels occur in the noble gases, the halogens can additionally store thermal energy on the rotational and vibrational energy levels. All particles / quantum objects capable of chemical bonding can interact with each other electromagnetically. Since such objects are usually composed of both positive and negative charges, there are always both repulsive and attractive forces, ie, interactions which can increase or decrease the distance of the particles, depending on which component is stronger. At a relatively large distance, the attractive effect always predominates, and the repulsive effect at very small intervals. In between there is a distance, namely the equilibrium distance, at which both effects compensate each other. If the particles are at equilibrium, a force-free state is present. The equilibrium distance is also called the bond distance, but from the force-freedom of this state one can not now conclude that no forces would occur in the chemical bond. If this distance is reduced by any external influence, the repulsive forces increase and try to prevent or mitigate the external influence. If the distance is increased by external influence, the attractive forces become larger and try to keep the external influence small. The binding system always strives to return to equilibrium. The bonding forces are therefore more clearly denoted as restitution forces. They ensure that particle ensembles form an inner cohesion as well as that the matter does not completely collapse. In everyday life, both repulsive and attractive effects of chemical bonding are of equal importance. If we use natural or artificial stones to build houses, cathedrals or even bridges, we use the repulsive effect of chemical bonding. If we put horses or oxen on the drawbar of a wagon, when we moor boats or ships in the harbor with ropes, we use the attractive forces of the particles in the drawbar or ropes. Large restoring forces are generally found when the equilibrium distance is short. Since large restoring forces, according to the rule of force, lead to large distances between the energy levels and subsequently to small entropies, we can see a link between the entropy and the chemical bond: strong bonding means small entropy. However, this relationship is initially only valid for the vibrational part of the entropy. 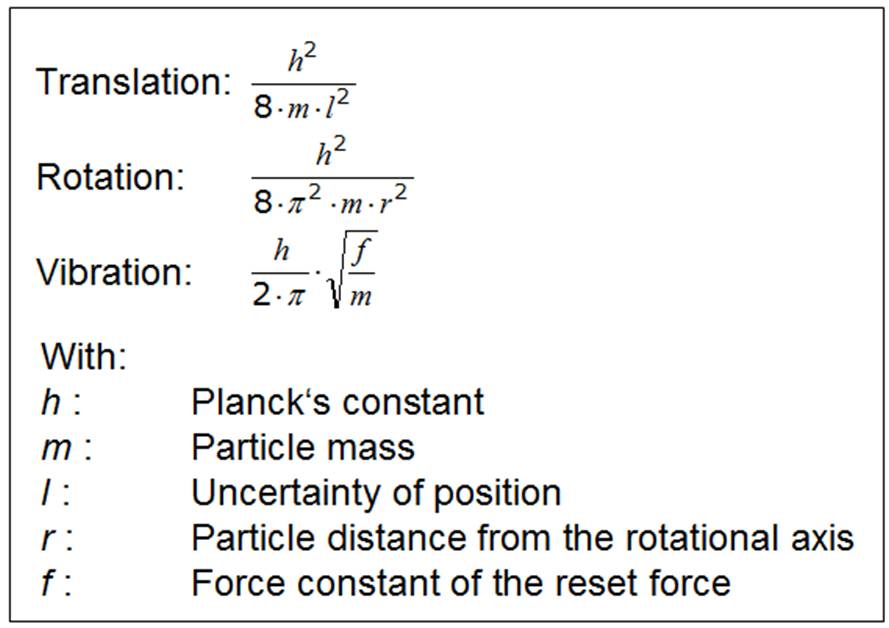
The large restoring forces enter into the counter of the vibration formula over the force constant f, resulting in large level distances and as a consequence a small proportion of vibrational entropy at standard temperature. Although we proceed from negligible forces between the particles in the noble gases, similar conditions still exist. As a rule, the gas particles are trapped in a container and are subjected to compressive forces therefrom from the vessel walls. These have a direct effect on the translational enegy levels. If the compressive forces are increased to the gas, the distances between the translational energy levels become larger. If gases are not held in a container by the walls but, for example, by gravitation as in planetary atmosphere, we know that there is a hydrostatic pressure in the gas atmosphere. Therefore, in the lower layers, larger compressive forces occur than in the higher layers. This also has an effect on the level intervals, which are also no longer equal in the entire gas space. |