|
The halogens bromine and iodine are not gaseous at 298 K. Nevertheless in thermodynamical tables the entropy values are listed for both, gaseous bromine and iodine, at 298 K and so we are able to compare the all gaseous halogens very well with the noble gases. The elementary groups behave very similar, for their entropy values increase within the group because of the increasing mass of the atoms and/or the molecules. However the curve of the halogens is altogether at lower values. From this fact we recognize that the motion of each halogen atom is reduced by the force of the nonpolar atomic bond. According to the force rule larger forces lead to smaller entropies. Mass and force rule make these phenomena understandable. A detailed discussion on the forces important for the substance properties 'Entropy' can be found here: |
|
5.2.2. The Alkali Metals and Alkaline Earths in Comparison
|
|
|
|
5.2.3. The Main Groups of the Periodic Table of Elements The atomic entropies of the main group elements are shown in the following chart. The values apply to 298 K and 1013 hPa. 
Some of the data represented here have already been published in: A. Jungermann, "Das Kellerregalmodell für Entropie und Enthalpie". Praxis der Naturwissenschaften, Chemie 48(5)2000 |
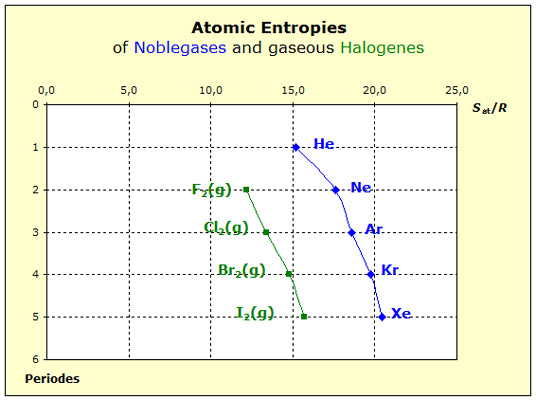 The chart on the left shows that the entropies of the noble gases increase from period to period. Since we assume the noble gas atoms to exert only weak forces to each other, the in- creasing mass will be responsible for the en- larged entropy values within the elementary group of the noble gases. The only forces effecting on the noble gas atoms are coming from the walls of the system and we consider them to be constant as long as the conditions of tem- perature and pressure remain constant.
The chart on the left shows that the entropies of the noble gases increase from period to period. Since we assume the noble gas atoms to exert only weak forces to each other, the in- creasing mass will be responsible for the en- larged entropy values within the elementary group of the noble gases. The only forces effecting on the noble gas atoms are coming from the walls of the system and we consider them to be constant as long as the conditions of tem- perature and pressure remain constant. 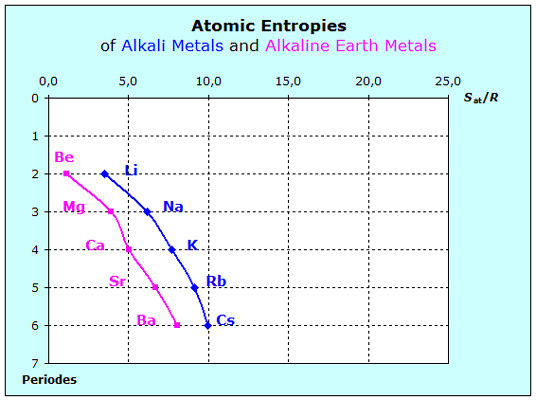 At first sight the diagram on the left looks very familiar with its two curves running nearly parallel from period to period to greater values. However, the in- creasing mass is only partially responsible for the increasing entropy values. Since with increasing period atomic trunks become larger and the dis-tances between the metal atoms in the lattice decrease. Thus the forces become less strong and the entropy additionally increases.
At first sight the diagram on the left looks very familiar with its two curves running nearly parallel from period to period to greater values. However, the in- creasing mass is only partially responsible for the increasing entropy values. Since with increasing period atomic trunks become larger and the dis-tances between the metal atoms in the lattice decrease. Thus the forces become less strong and the entropy additionally increases.