We start our discussion of compounds with the non-metals and try to compare the hydrogen halides with the halogens. Both kinds of substances consist out of diatomic molecules and the tables contain the
standard entropies for both in gas condition. This makes the comparison simple. |
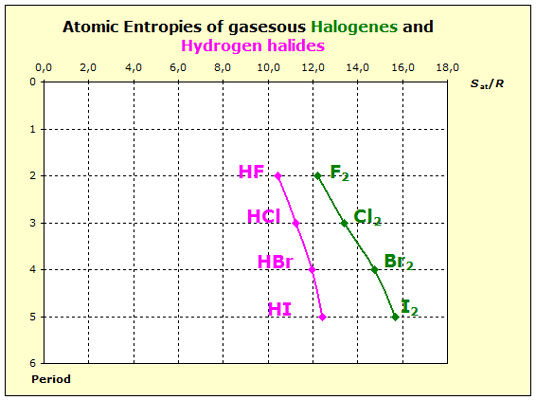 Two differences occur: Two differences occur:
1. Different kinds
of chemi- cal bonding.
2. Different particle mas- ses.
Hydrogen halides contain polar bonds and this leads to stronger intermolecular forces and to lower entropy values than with the corresponding nonpolar pure halogens
Hydrogen atoms have smaller masses than halogen atoms, so the hydrogen halides have smaller masses than the respective pure halogens.
Both, force and mass effects cause that the entropy values of the hydrogen halides are smaller than those of the pure gaseous halogens.
|
We now focus on simple compounds of the metals as salts, e.g.
the chlorides of the alkali-metals and alkaline-earth-metals. Some of these substances are
well-known like sodium chloride. Some others - such as
potassium chloride or magnesium chloride - we know from scientific school lessons. All these substances are solid and crystalline under standard conditions. The crystals are hard and
brittle. Because of this characteristic sodium chloride is also called rock salt. This property indicates large forces and smaller entropies rather than
the gaseous materials discussed above. |
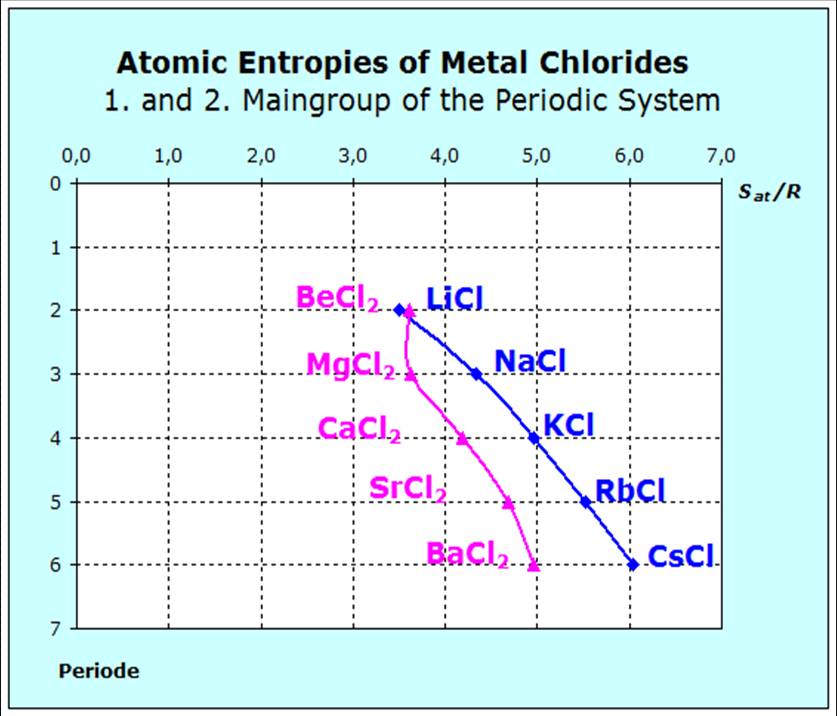
Again we will have to answer two questions:
1. Which forces arise?
2. How large are the particle masses?
Because we already learned to deal with atomic entropies, we are able to compare substances with different
stoichiometry.
Since the alkaline-earth atoms form doubly charged ions, we expect larger forces than with the chlorides of the alkali metals and thus smaller entropy values. The pre- sented diagram, however, shows one exception: Beryllium chloride |
In order to understand this deviation, we regard two further
diagrams. One shows the differences of the electronegativity of these
salts and the other one the bonding distances. |
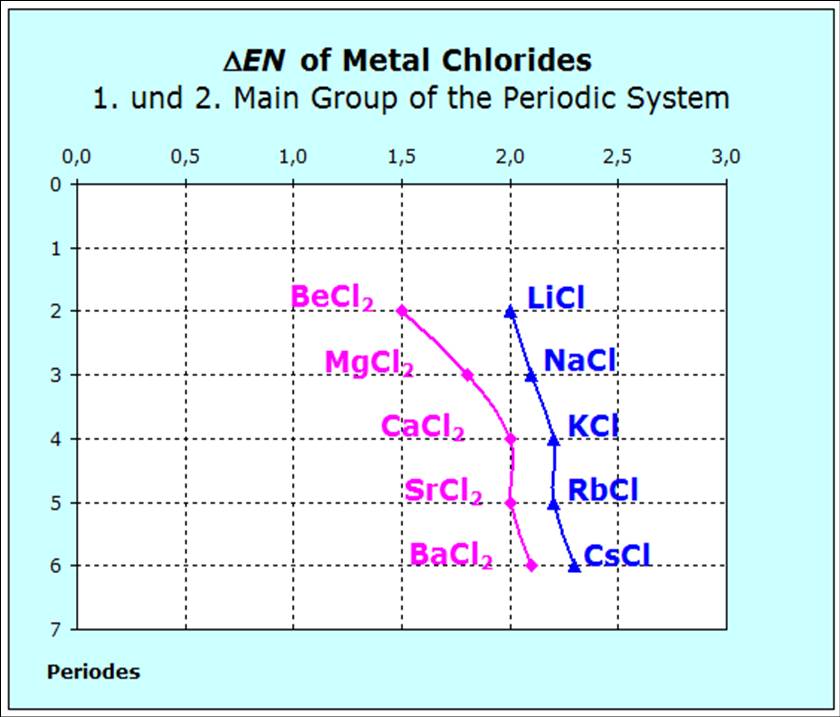 For the chlorides of the 2. main group we of course find the differences of the electronegativity to be smaller than those of the alkali metal chlorides. For the chlorides of the 2. main group we of course find the differences of the electronegativity to be smaller than those of the alkali metal chlorides.
With the beryllium chloride the value with 1,5 is already so small that we no longer speak of an ionic bonding. This causes smaller forces and there-fore a larger entropy value.
Since the masses of the alkaline-earth atoms are approx. 9% larger than those of the alkali metals, the influence of the mass is not so important, and the smaller entropy values are mainly a consequence of the larger forces. |
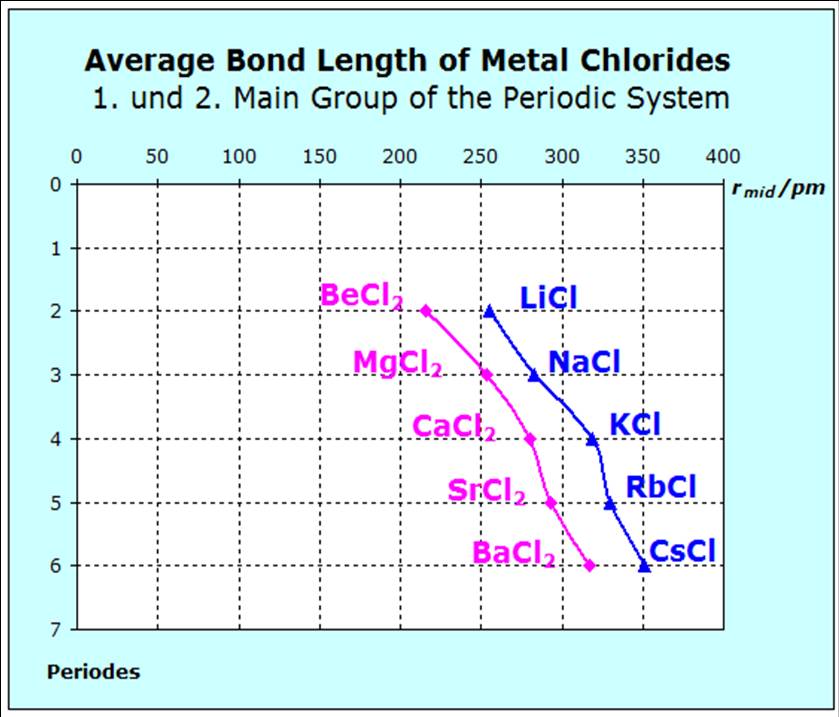
The diagram showing the different bonding distances confirms the given inter-pretation of the entropy values
including the exception of the beryllium chloride. The bonding distance is
relatively large in comparison with the other substances, there-fore the
forces are un-systematically small and thus the entropy value is found to be large. |
 Two differences occur:
Two differences occur:
 For the chlorides of the 2. main group we of course find the differences of the electronegativity to be smaller than those of the alkali metal chlorides.
For the chlorides of the 2. main group we of course find the differences of the electronegativity to be smaller than those of the alkali metal chlorides.