6.1. Thermal Work
If work in a physics sense is performed on a substance, the energy state of the substance changes. This process is commonly referred to as work in a scientific sense and the type of process can be described by means of adjectives such as electrical, mechanical or the like for a more precise definition.
Video with soundtrack: Thermal work and photons The decisive criterion for the fact that an operation receives the attribute "thermal" consists, therefore, in the constancy of the entire quantized energy storage system, or, as quantum theoric, the maintenance of the eigenvalue system. All energy levels remain the same, but the occupation numbers change. |
|
6.1.1. Substances with Equal Stoichiometry In section 3.2, we have already dealt with the fact that the entropy of a substance at a temperature T is linked to the amount of energy that is needed to heat the substance from the absolute temperature zero point to the temperature T. However, since this overall energy is not accessible to us by simple means, the following experiments are designed in such a way that we can see what effect this total energy has on the thermal behavior of a substance at any temperature. If we heat two substances of different total energies with the same amount of thermal energy, we can examine whether they behave differently in the ratio of their entropies. In the concrete planning of such an experiment, however, questions arise that must be considered: |
|
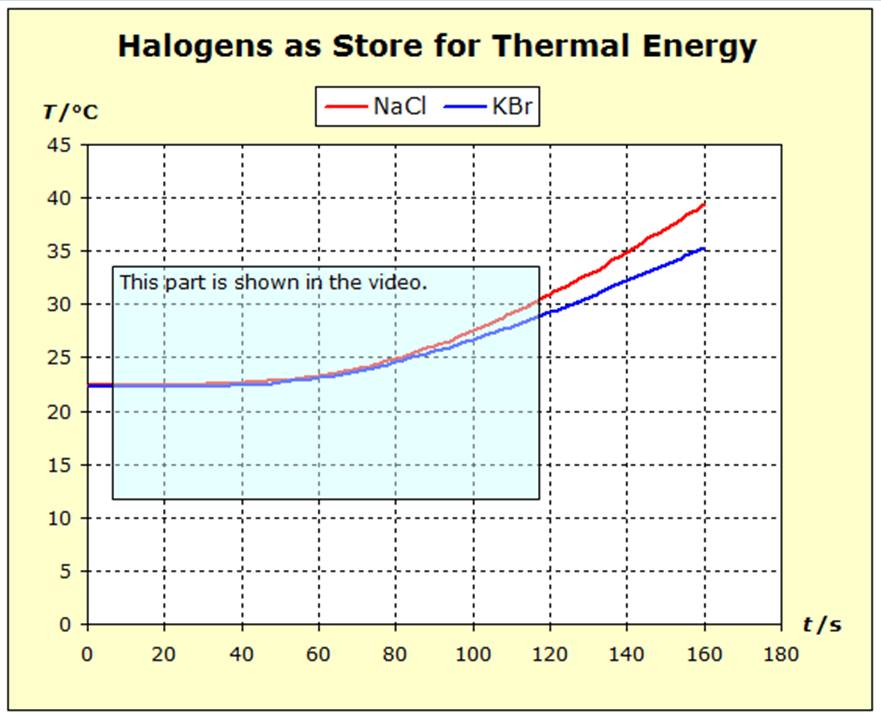
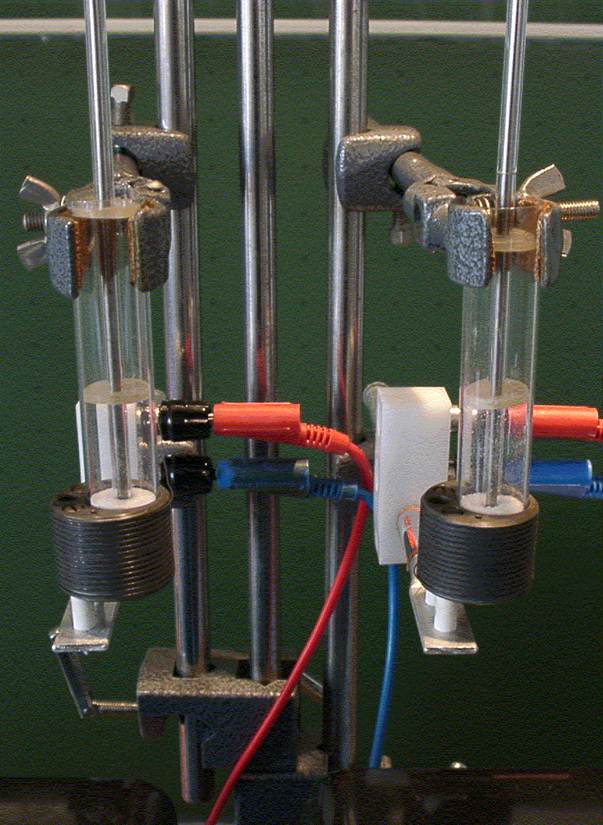
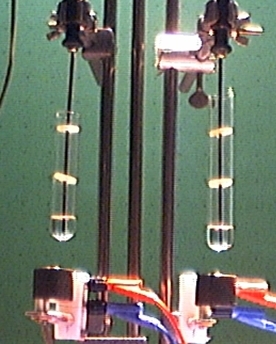
From the following figure it can be seen that two test tubes are placed in two electric heating blocks which are filled with two small-crystalline sections of material so that the fillings are flush with the upper edge of the heating blocks. In the left heating block, sodium chloride should be heated, in the right potassium bromide. |


|
From the considerations of the atomic entropy (see Section 5.2), the same energy supply should be applied to the same atomic numbers. So we have to consider the different densities of the two substances. In the left test tube a quantity of n(NaCl) = 75.2 mmol of sodium chloride and in the right test tube n(KBr) = 47.1 mmol of potassium bromide are heated. |
Video: Thermal work executed to halogenides
|
|
After the voltage is switched on, the heating blocks are first heated and after about 25 seconds the centrally placed temperature measuring probes react to the thermal energy supplied. S(NaCl) = 72.13 J/(Kmol) or S/R = 8.68 S(KBr) = 95.90 J/(Kmol) or S/R = 11.53 This value is comprehensible both from the force and the mass rule: |
|
* If you would like to find out more about the "thermal inert" property, please refer to the following link: To define thermal inertia |
|
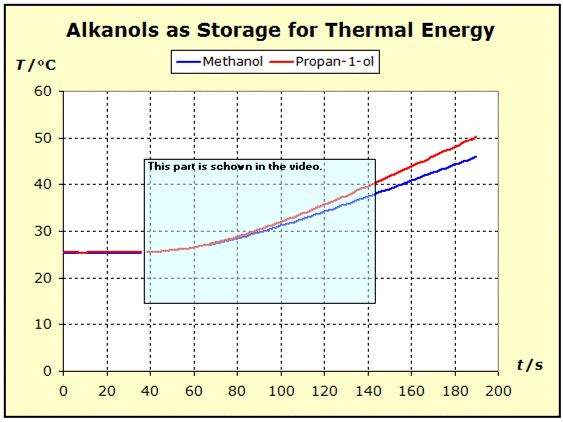
6.1.2. Substances with Unequal Stoichiometry If the thermal properties of substances with different stoichiometry are to be compared, it has already been pointed out in section 5.2 that it is expedient to consider the values of the entropy and heat capacity, in relation to the number of atoms. The importance of the atomic entropy will be explained here by a simple example. ST(1mol N) = 141.6 J/K or ST/R(1mol N) = σT(1mol N) = 17.03 = σtot(1mol N) Since these individual atoms would neither be able to rotate nor to vibrate in the gas, only the levels of the translational states would be occupied in this gas. The program Thermulation-II shows that this one mole of N atoms is distributed to 4260 levels. We assume three eigenvalue systems - one each in the x, y, z direction - on the levels of which 1 mol of N atoms are distributed. In each direction 1420 levels are occupied: 3x1420 = 4260. The above entropy value results from this distribution. σT(0.5mol N2) = 9.03 σR(0.5mol N2) = 2.82 σV(0.5mol N2) = 0,00 The two σtot values represent comparable values of the thermal inertia of nitrogen atoms and nitrogen molecules because they refer to equal numbers of atoms. We simplify now the spelling of atomic entropies: σat(N) = 17.03 σat(N2) = 11.85 In many everyday cases, however, a good estimate of thermal material properties is desired, and this can be easily achieved by forming the atomic entropies Sat or Sat/R = σat from the tabulated standard entropies by dividing the standard entropies by the number of atoms of the corresponding molecule type. The next experiment shows the thermal properties of methanol and propan-1-ol in comparison. In the interpretation of the experimental results, the atomic entropies of these two compounds help us very well to understand the cause of the thermal behavior via the force and mass rules and the atomic structure of these two substances. |
|
The measuring apparatus (see picture below) is constructed similarly to the previous experiment. The current and voltage measurement is carried out directly on the DC power supply. Here, too, the current intensity is set so that both substances per atom receive the same energy supply per unit of time. 
 |
Video: Thermal work on primary alkanols
|
|
The experimental results show that an equal energy supply to propan-1-ol results in a higher temperature increase than in the case of methanol, which thus proves to be the thermally more inert substance. If one considers the entropy values of the two substances, it follows that the molar standard entropy is smaller for methanol than for propan-1-ol: S(CH3OH) = 126.8 J/(Kmol) or S/R = 15.25 = σ(CH3OH) S(C3H7OH) = 192.9 J/(Kmol) or S/R = 23.2 = σ(C3H7OH) From these values, however, the thermal behavior of the two substances of the experiment shown above is not readily understood. However, if we calculate the atomic entropy values from the two values, the image changes: Sat(CH3OH) = [126.8 J/(Kmol)] : 6 = 21.13 J/(Kmol) or Sat/R = 2.54 = σat(CH3OH) Sat(C3H7OH) = [192.9 J/(Kmol)] : 12 = 16.05 J/(Kmol) or Sat/R = 1.93 = σat(C3H7OH)
Sat(NaCl) = [72.13 J/(Kmol)] : 2 = 36.07 J/(Kmol) or Sat/R = 4.34 = σat(NaCl) Sat(KBr) = [95.90 J/(Kmol)] : 2 = 47.95 J/(Kmol) or Sat/R = 5.77 = σat(KBr) Let's try a rough estimate. For this we dispense with the differentiation between the two salts and the two alcohols and take the respective mean values as points of reference and we analyze the four measurements as follows: n(NaCl) = 150.4 mmol ions; n(KBr) = 94.2 mmol ions; n(meanvalue) = 120 mmol σat(salts) = 5 (approx.) The experimental data were: Salts: Δt = 100s; ΔT = 7K; I = 2.5A Alkanols: Δt = 100s; ΔT = 13K; I = 1.5A Although the apparatus and the measurements were simple, the results of the measurements are consistent with the material specifications and the salts confirm themselves as the thermally more inert substances compared with alkanols. |