7.2. The Chemical Equilibrium
In publications and thermodynamic tables, not only the standard values of enthalpy and entropy of the formation reactions are listed, but also values for the reaction enthalpies of specific reactions, such as combustion reactions. At the same time, reaction enthalpies of this type are referred to as combustion enthalpies or calorific values in analogy to the formation enthalpies of individual substances. For example, the reaction equation for the combustion of natural gas is given here: If such values are then used for the assessment of a substance such as natural gas as a primary energy carrier, this approach raises questions: Is the substance natural gas an energy carrier? Who carries what energy? What are the energetic functions of oxygen and / or the resulting products in combustion? 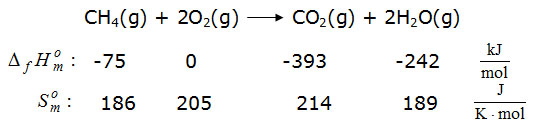
 This step urgently requires a comment with regard to the questions posed, since these values express the influence of all the substances involved. On the other hand, this value no longer depends on whether it was formed from two or more substances. He could as well represent only one substance. The values on the reactant side characterize the thermal properties of the reactants and those on the product side correspond to those of the products. Illustratively, one can say that the sum values of the reactant side represent a mean value with imaginary mean value particles and the sum values of the product side characterize in the same way a developing fictive end product. This view also appears to be justified because all reactions in which the values for the reactant and product side are the same behave thermodynamically equivalent. 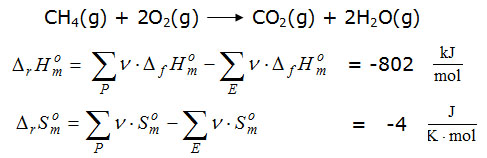 By this computational approach, the reaction equation simplifies to a reaction which leads from the "mean value E" (educt) with prototypically conceived median particles to a "mean value P" (product) with other, also prototypically imagined median particles. This view characterizes the actual thermodynamic quality of the investigated reaction better than the description "combustion enthalpy" of a substance arbitrarily selected from the reaction mixture. In two video sequences it is shown that the equilibrium is established both by the starting side and by the product side in spontaneous processes. |
Video with Sound: Spontaneous Endothermic Path to Equilibrium, Forward Reaction |
One can very impressively observe how the drive for the spontaneous formation of the equilibrium decreases from very large values to zero in about 90 seconds. The temperature drops by 3K. |
Video with Sound: Spontaneous Exothermic Path to Equilibrium, Back Reaction |