If equal volumes of ethanol and water are mixed, the final volume is smaller than the sum of the two initial volumes. Very often, attention is drawn to this phenomenon without mentioning, that this process is exothermic. In the following series of experiments the first three members of the homologous series of unbranched monovalent alkanols and additionally propan-2-ol are investigated systematically.
After you have watched the video, we first put the results here:
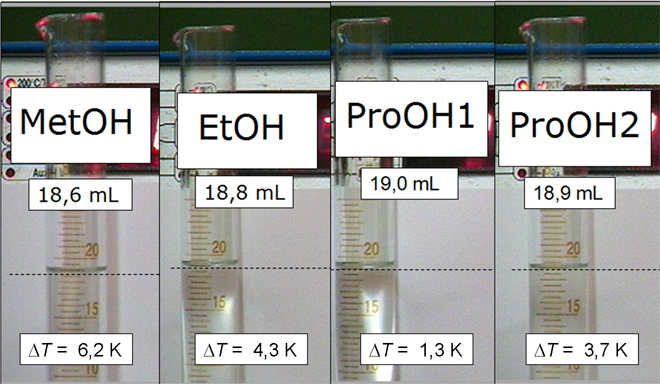
In the interpretation of this series of experiments, the first step is to understand the exothermic and the volume reduction. Furthermore, there are also questions on equilibrium theory, the theory of bonding, and the chemical reaction.
A rule of thumb states that a reaction is exothermic when the number of polar bonds or the polarity of bonds is increased. The cohesion in water is more strongly based on hydrogen bonds than in the primary alcohols, for example because two free electron pairs as well as two bridging H atoms are present in the water, while in the case of the alcohols, two free electron pairs, but only one bridging H atom per molecule. Each water molecule can have a maximum of four hydrogen bonds, whereas the alcohols have the upper limit in the temporal and spatial mean at two hydrogen bonds per molecule.
If water and alcohol come together as molecules, the upper limit is increased for the alcohols by the greater supply of bridging H atoms of the added water molecules. The upper limit for the water molecules, however, remains at four bridges per molecule. This results in a larger number of polar bonds, which, according to the rule of thumb, explain the exothermic effect.
In order to facilitate the understanding of this volume reduction, we should once more focus on the considerations concerning the bonding forces. (Considering chemical bond phenomena)
Assuming that the bond lengths of the CC, CH, CO, and OH bonds remain approximately constant when the alcohol and water are mixed, the reduced volume should be related to the chemical bonds between the molecules, that is, the van-der-Waals bonds between the temporary and stationary dipoles and the hydrogen bonds. The fact that the alcohols are involved by increased H-bonds has already been mentioned above. The temporary dipoles of the alcohols make an additional interaction with the stronger dipoles of the water molecules, which leads to the force equilibrium of the restoring forces only at smaller intermolecular distances.
This chemical reaction, which clearly changes the bonding ratios, also shows phenomena of an equilibrium reaction. If the different exothermies are compared in the test series with the different chain lengths, this temperature effect becomes stronger with decreasing chain length. In this direction, however, the amounts of the educts also increase. The starting amount of substance were 130 mmol of the propanols, 172 mmol of ethanol and 247 mmol of methanol. Although the larger amount of substance does not directly affect the value of the equilibrium constant, the additional amount of substance reacts exothermically to the products according to Le Chatelier's principle. This enables us to understand the different temperature effects within the test series.