7.4. Thermodynamic Drive for Chemical Reactions
The purpose of this section is to show that the drive for a chemical reaction can be traced back to the same basic phenomenon as the drive which leads to the temperature compensation between a hot and a cold substance, as presented in section 6.3. The four basic questions to the drive remain relevant in this section as well, and at the end of this chapter we will see that the answers are in principle the same. |
Video with sound: model attempt to establish an equilibrium, forward reaction |
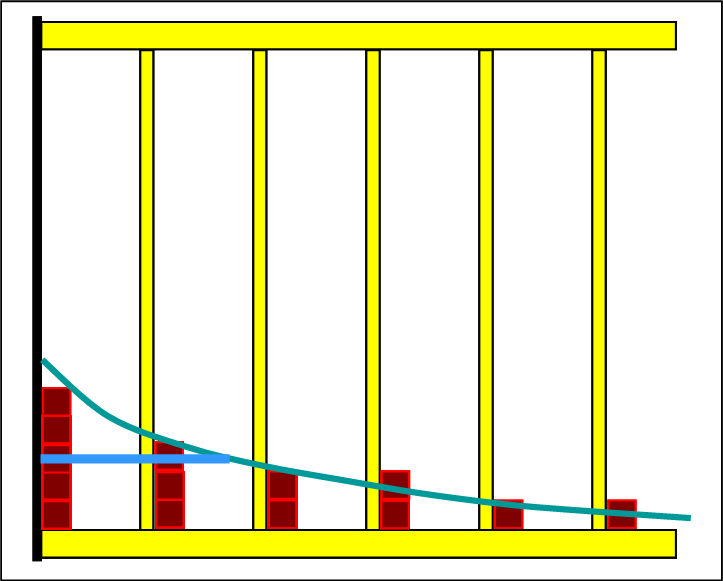
The following two pictures show the initial state of the reactants and the final state of the set equilibrium. Since the lines of the half-value energies are sketched, it is easy to see that the equilibrium has set itself on an endothermic path. |
|
 |
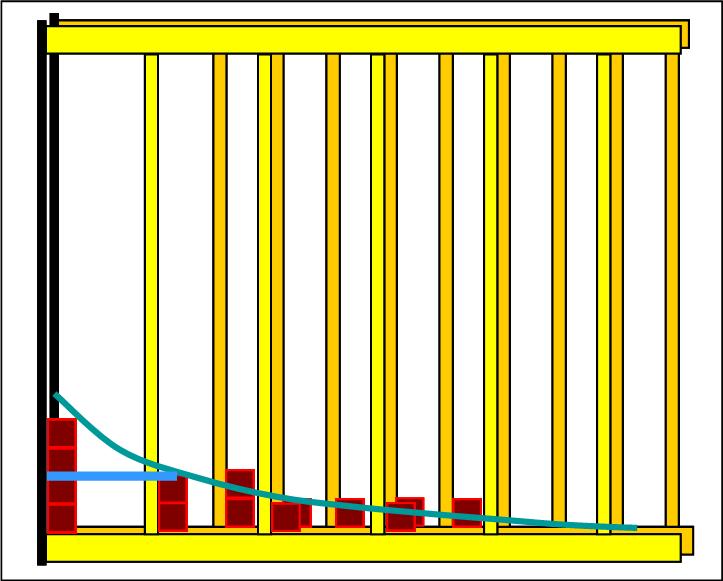 |
On the reactant side, 6 levels are occupied and in equilibrium the particles are distributed to 9 levels. This is an indication that the entropy has increased. The way in which the product shelf is filled up in the individual steps and removed from the reactant shelf corresponds precisely to the basic rule. Each new distribution is "Boltzmann-like". With the Thermulation-I program, it can be shown that the half-value energy of reactants and products is the same in every step. |
|
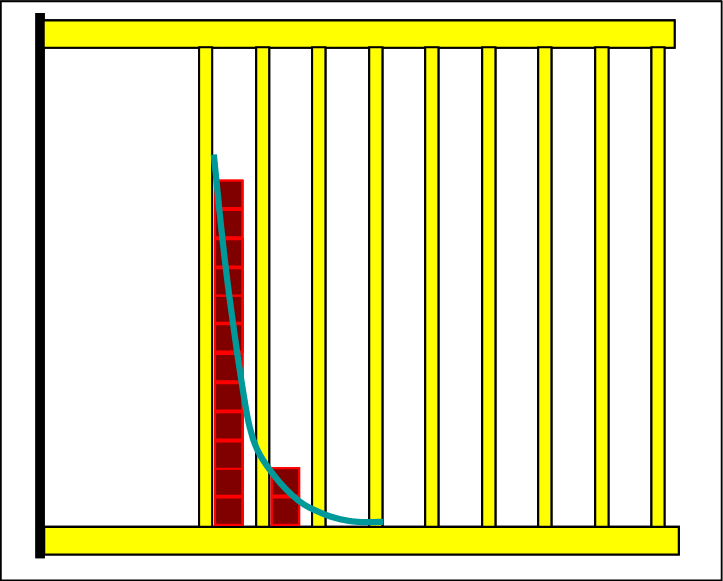 |
Since now the lowest level is clearly above the zero level of the total system, the particles are compressed to the two lowest levels of the product side because the total energy is too small to occupy even higher levels. This state would not spontaneously and voluntarily be reached from the reactants. There only two energy levels would be occupied, indicating a low entropy. Our experience teaches us that the natural processes end at maximal entropy. |
But once the final substance has been obtained by other means, it can be cooled to this temperature. Then one can establish that this substance spontaneously and voluntarily takes the exothermic reaction, of course only if there is no kinetic inhibition. This case can be discussed with the following picture:
|
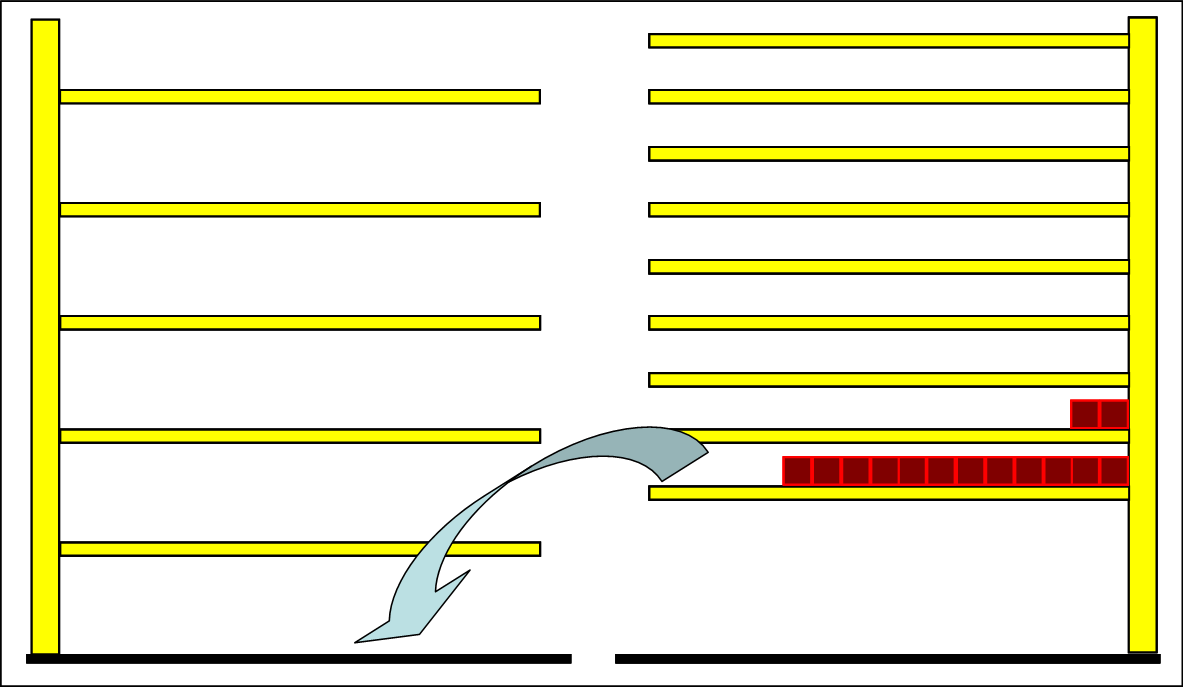 |
If particles fall down during the reaction, some have to be raised to higher levels because of energy conservation. This is achieved by the photons that are emitted during the transition to the lower levels. The photons do not leave the system because of the isolation, but instead bring their own system particles to higher levels. The particles are distributed to more than two levels, and the half-value energy, ie the temperature rises. The back reaction is exothermic. |
At the end of the section on the drive for chemical reactions we want to check again how we can now answer the same four questions about the drive from section 6.3.
 |
We can now summarize the answers to the four questions on the thermodynamic drive in temperature equilibrium and chemical reaction: |